
IV Ketamine
IV ketamine can be a powerful option for those struggling with persistent pain, depression, or anxiety — especially when traditional treatments haven’t helped. We use a safe, low-dose protocol designed for both physical and emotional relief.

SPRAVATO® for Depression
SPRAVATO® (nasal esketamine) may help patients with treatment-resistant depression who haven’t responded to standard antidepressants. It can also be beneficial for those whose depression is associated with severe anxiety. Administered in a calm, supportive setting with medical supervision.
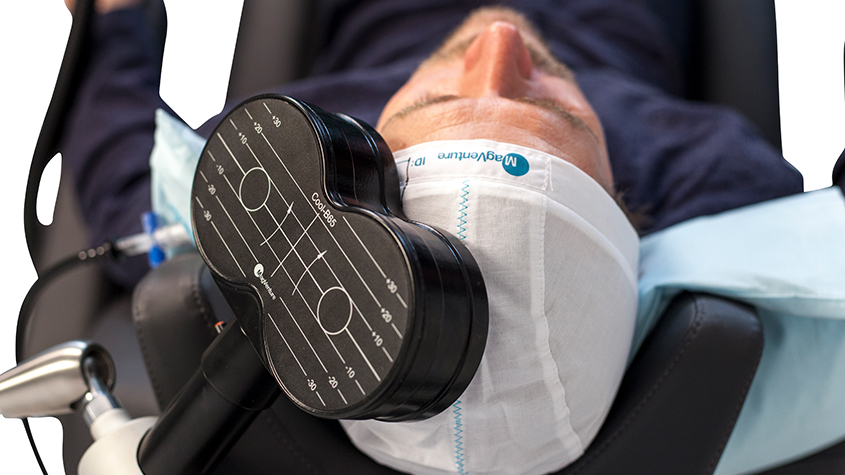
Transcranial Magnetic Stimulation (TMS)
TMS is a noninvasive treatment that uses magnetic pulses to stimulate specific areas of the brain involved in mood regulation. It's FDA-approved for treatment-resistant depression and has shown promise in reducing symptoms of anxiety, PTSD, and chronic pain.

Medical Evaluation & Care Planning
Your care starts and continues with in-depth, one-on-one visits that explore both physical and emotional contributors to your symptoms. Whether you’re dealing with chronic pain, anxiety, depression, or stress-related conditions, we take time to listen, connect the dots, and follow a plan that supports both your body and mind.
SPRAVATO, TMS, And IV KETAMINE COMPARISONS
| Categories | Spravato | TMS | Ketamine |
| Mechanism of Action | Esketamine, the S-enantiomer of ketamine, acts as an NMDA receptor antagonist, modulating glutamate neurotransmission. | TMS uses electromagnetic pulses to stimulate specific brain regions, primarily the left dorsolateral prefrontal cortex (DLPFC). | Ketamine, an NMDA receptor antagonist, affects glutamate transmission, potentially enhancing synaptic plasticity and alleviating depression and pain. |
| Administration | Self-administered via nasal spray under medical supervision by REMS-certified prescriber. Monitored in-office for 120 minutes. | Non-invasive treatment involves placing a magnetic coil on the scalp to deliver pulsed magnetic stimuli to the brain. Takes less than 30 minutes. | Administered intravenously (IV) over 1 to 3.5 hours, depending on the condition being treated. |
| Treatment Schedule | Twice weekly for the first 4 weeks, followed by once weekly for 4 weeks, followed by maintenance dosing. | Five days a week for about 6 weeks. | Variable, generally a series of 8 infusions for chronic pain and 4 infusions for depression. |
| Potential Candidates | Patients with Major Depressive Disorder, single episode or recurrent, moderate to severe. Failure of 2+ oral antidepressants. | Patients with Major Depressive Disorder, single episode or recurrent, moderate to severe. Failure of 2-3+ oral antidepressants. Participation in psychotherapy. | Patients seeking viable alternatives to conventional treatments for depression, Complex Regional Pain Syndrome (CRPS), and other types of chronic pain. |
| Insurance | Generally authorized by insurance; coverage and out-of-pocket expenses vary by plan. | Generally authorized by insurance; coverage and out-of-pocket expenses vary by plan. | Unlikely to be covered by insurance. |
| Side Effects | Common side effects include dissociation, dizziness, nausea, sedation, anxiety, increased blood pressure, and feeling "drunk". | Common side effects include headaches, scalp discomfort, lightheadedness, facial muscle twitches, tingling. Rarely (0.1%), TMS may trigger transient seizures. | Common side effects include dissociation, dizziness, nausea, sedation, anxiety, increased blood pressure, and feeling "drunk". |
| Drive Self to/from Treatment | No | Yes | No |


